A promising new method of targeting cancer cells takes advantage of the fact that the same mutation cascades that lead to cancer often damage the cell’s innate antiviral pathways. This can then leave those cells susceptible to viruses that would be harmless to healthy cells. Therapeutically, infection with one of these oncolytic (cancer-killing) viruses would result in the death of infected cancer cells, with the additional benefit of triggering the immune system to recognize antigens associated with the cancer itself and eliminate any remaining uninfected cancer cells throughout the body.
One example of this that’s currently being studied here at Arizona State University is the myxoma virus — a virus that is lethal in European rabbits but completely nonpathogenic in all other host species. While wildtype myxoma is able to infect many cancer cells, several types are more restrictive of viral replication, an issue that needs to be better-understood and potentially corrected before myxoma could be used therapeutically.
In a recent publication, Dr. Masmudur Rahman from the McFadden lab examined in-depth a specific pathway that appears to regulate the efficiency of the myxoma virus replication within cancer cells: DHX9 and the previously uncharacterized antiviral granules it can trigger within the cytoplasm of infected cells.
DHX9 is a large multi-functional protein implicated in both oncogenesis and tumor-suppression; additionally, while it is used by several viruses as an essential part of their replication process, it is also involved in the innate immune response against other viruses.
In human cancer cells infected with myxoma, Rahman and his collaborators showed that DHX9 locates in the cytoplasm and binds to viral RNA transcripts, preventing viral protein synthesis and mitigating the effects of infection on the cell and the overall cancer. This figure from their paper shows the co-localization of DHX9 and dsRNA into cytoplasmic granules (in myxoma-infected non-permissive pancreatitis cancer cells) quite clearly:
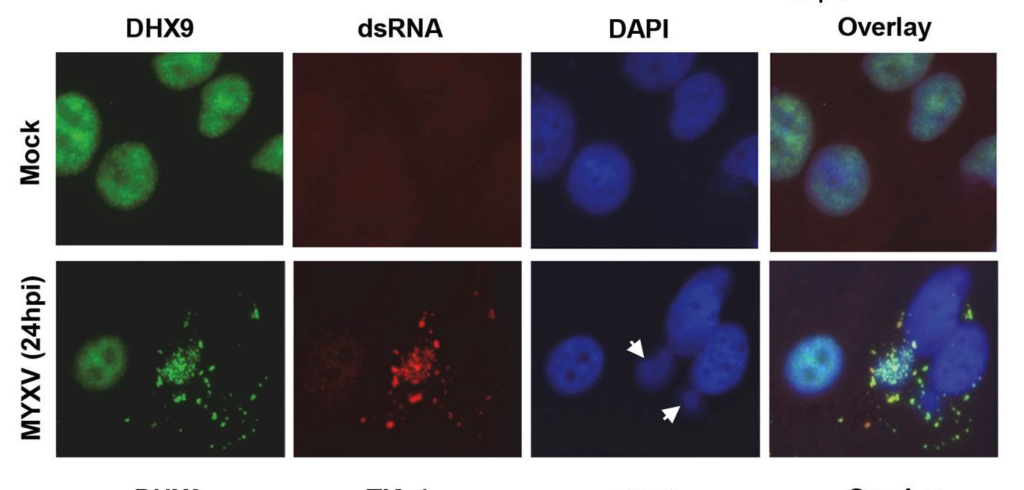
To quote their summary of the clinical importance of their finding,
“This is particularly significant because in human cancer cells that are nonpermissive for MYXV, for example, PANC-1 human pancreatic cancer cells, DHX9 knockdown alone increased MYXV replication by more than 2 orders of magnitude. This suggests that selective targeting of DHX9 can potentially enhance oncolytic virotherapy of MYXV, and possibly other poxviruses, in a subset of cells that are not naturally permissive for the virus.”
Rahman et al., 2021
What’s next? For now, the lab is looking into the genes and functional pathways that are expressed differently in these non-permissive infected cells than in cells with reduced DHX9 levels, as well as exploring in more depth the specific domain of DHX9 that is involved in the formation of these anti-viral granules — since it is such a large protein with a variety of roles, the ability to target a single functional domain instead of the whole protein is likely to reduce negative side-effects on non-cancerous cells. This has the potential to lead to life-changing research for cancer patients, and I’m excited to see what Rahman and his team publish next!
References
- Rahman, M. M., Gutierrez-Jensen, A. D., Glenn, H. L., Abrantes, M., Moussatche, N., & McFadden, G. (2021). RNA Helicase A/DHX9 Forms Unique Cytoplasmic Antiviral Granules That Restrict Oncolytic Myxoma Virus Replication in Human Cancer Cells. Journal of virology, 95(14), e0015121. https://doi.org/10.1128/JVI.00151-21
- Bell, J. C. (2018). How Oncolytic Virus Therapy Is Changing Cancer Treatment. Cancer Research Institute. https://www.cancerresearch.org/en-us/immunotherapy/treatment-types/oncolytic-virus-therapy, retrieved 21 Jun 2022
